← calculating moles from molarity Molarity calculate moles molaridad wikihow grams mol liters solute ways how to calculate moles of solute Solved calculate the number of moles of solute in each of →
If you are searching about Molarity to moles calculator - Calculatorway you've came to the right page. We have 35 Images about Molarity to moles calculator - Calculatorway like How to Calculate Molarity using Solute Moles | Chemistry | Study.com, Formula To Calculate The Moles - Printable Templates Free and also Molarity Formula: How to Calculate Molarity with Examples. Read more:
Molarity To Moles Calculator - Calculatorway
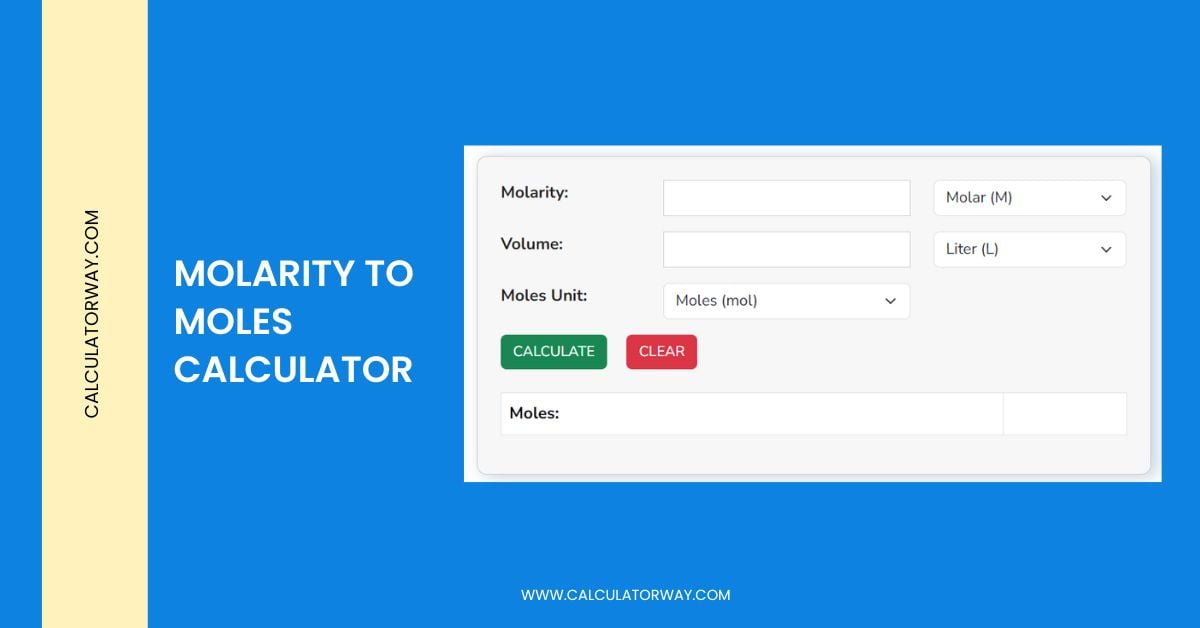 www.calculatorway.com
www.calculatorway.com
Solved M= Moles /L Determine The Molarity Of A Solution | Chegg.com
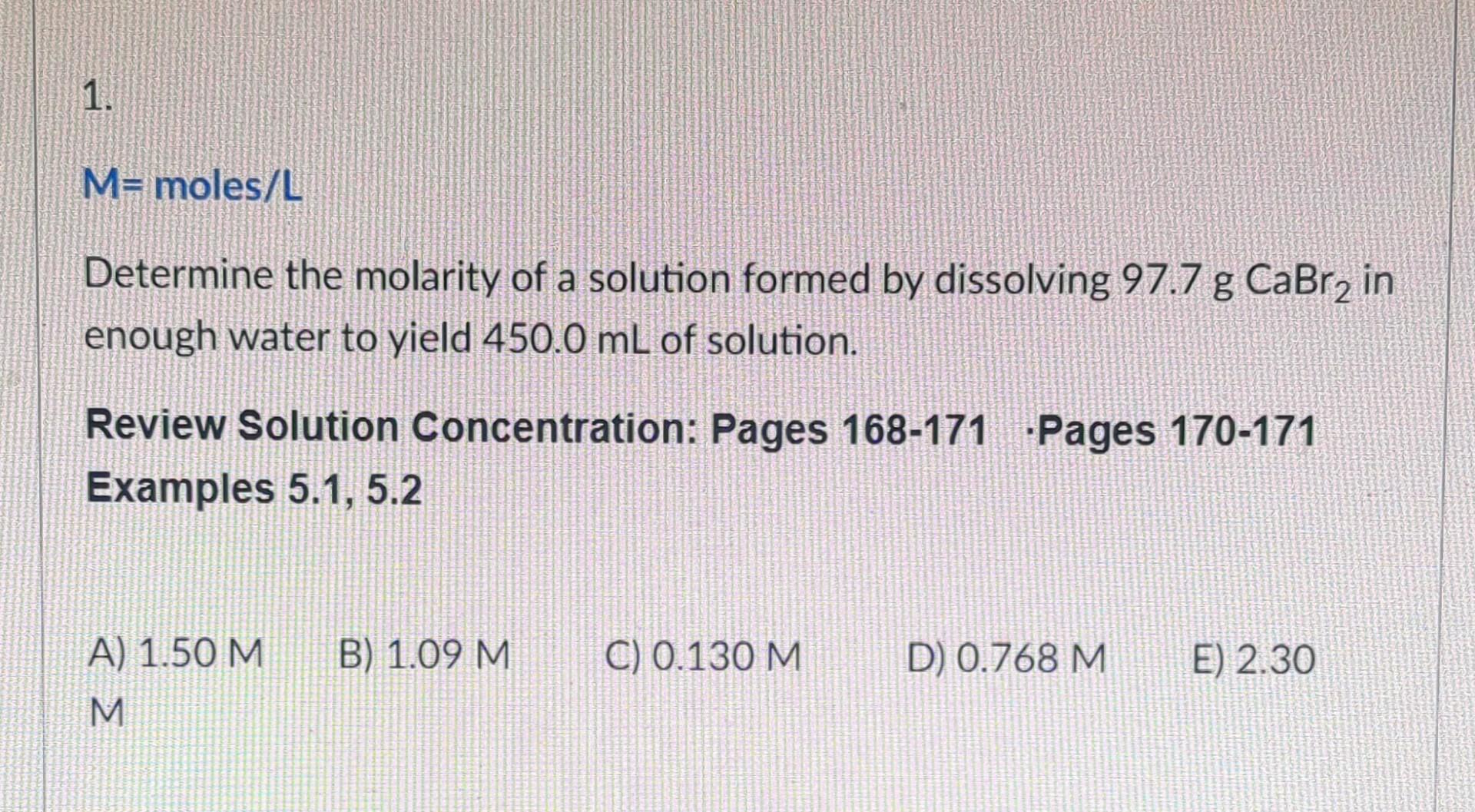 www.chegg.com
www.chegg.com
How To Calculate Molarity | Images And Photos Finder
 www.aiophotoz.com
www.aiophotoz.com
How To Calculate Moles Calculating Molarity, Solving For Moles & Grams
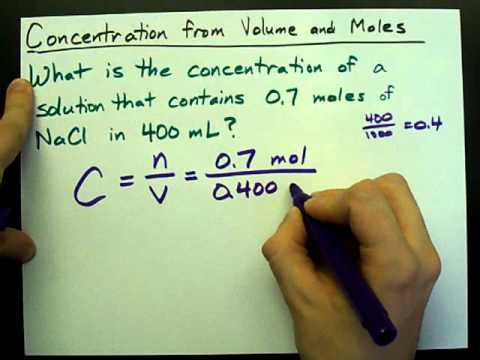 narra-howtoid.blogspot.com
narra-howtoid.blogspot.com
Calculate The Molarity Of A Solution Containing 5 - Answer: First
 www.studocu.com
www.studocu.com
Pharmaceutical Calculations | Moles And Molarity - Part 2
 pharmafactz.com
pharmafactz.com
molarity moles mol calculations between
Solved Determine The Molarity Of These Solutions Using The | Chegg.com
 www.chegg.com
www.chegg.com
Molarity Formula: How To Calculate Molarity With Examples
 www.wikihow.com
www.wikihow.com
molarity calculate moles mol calculations wikihow molaritas menghitung nacl
Moles Of An Element In A Compound Calculator
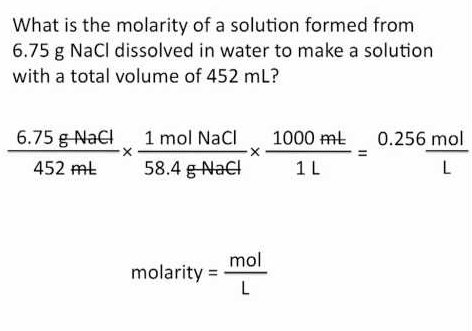 chatbotinc.com
chatbotinc.com
How To Find Moles? Molarity Calculation
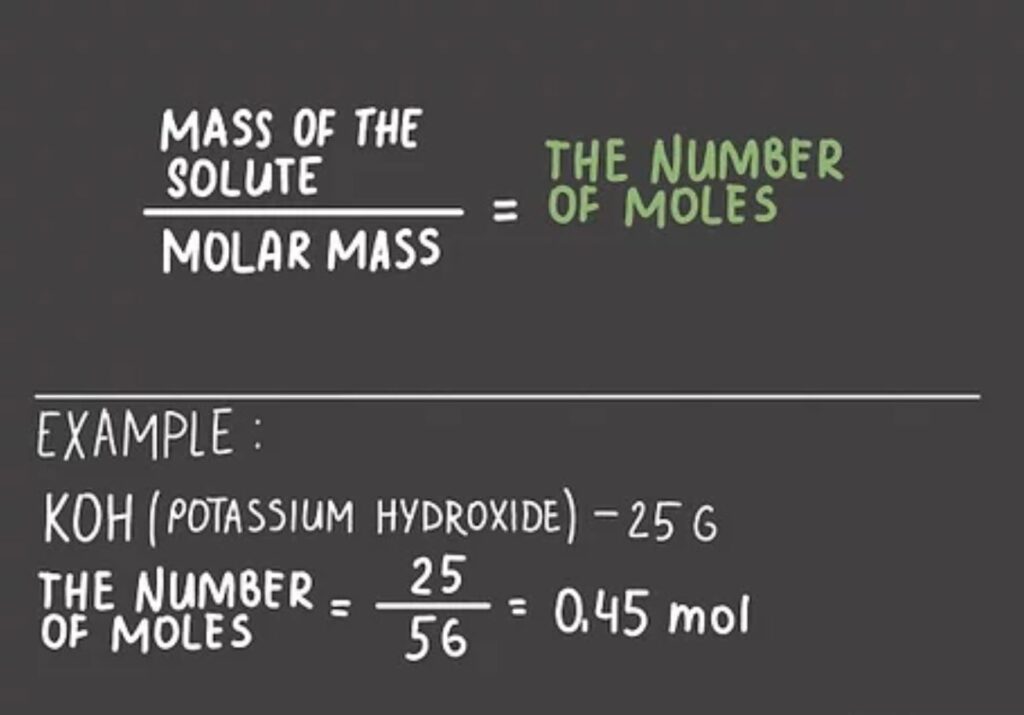 thestudyish.com
thestudyish.com
Calculating Molarity (given Moles And Liters) - YouTube
 www.youtube.com
www.youtube.com
moles given liters molarity
How To Calculate Molarity: A Comprehensive Guide To Understanding This
 www.supsalv.org
www.supsalv.org
Calculating Molarity - YouTube
 www.youtube.com
www.youtube.com
molarity calculating
How To Calculate Molarity Using Solute Moles | Chemistry | Study.com
Scientific Designing Of The Mole And Molar Volume Formula Triangle
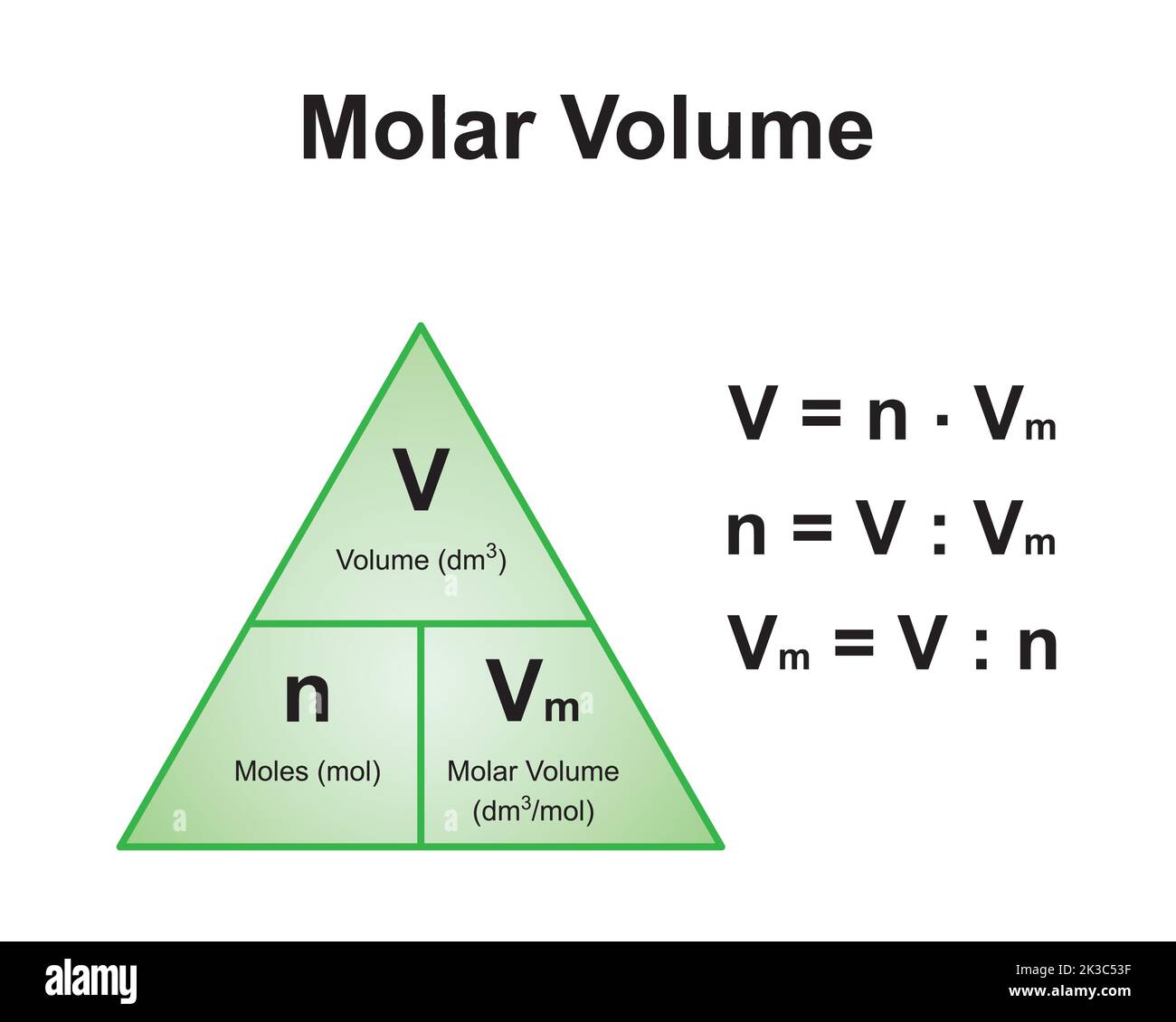 www.alamy.com
www.alamy.com
Formula To Calculate The Moles - Printable Templates Free
/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png) read.cholonautas.edu.pe
read.cholonautas.edu.pe
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters
 www.youtube.com
www.youtube.com
molarity grams moles volume liters stoichiometry chemistry solution dilution problems calculations
How To Calculate Molarity Using Solute Mass | Chemistry | Study.com
 study.com
study.com
Molarity | PDF | Mole (Unit) | Molar Concentration
 www.scribd.com
www.scribd.com
Solved Molarity = Moles/ Liter Using The Above Formula How | Chegg.com
 www.chegg.com
www.chegg.com
Molarity Formula: How To Calculate Molarity With Examples
 www.wikihow.com
www.wikihow.com
molarity calculate moles molaritas mass calculating berekenen mol menghitung van
Find Molarity From Mole - YouTube
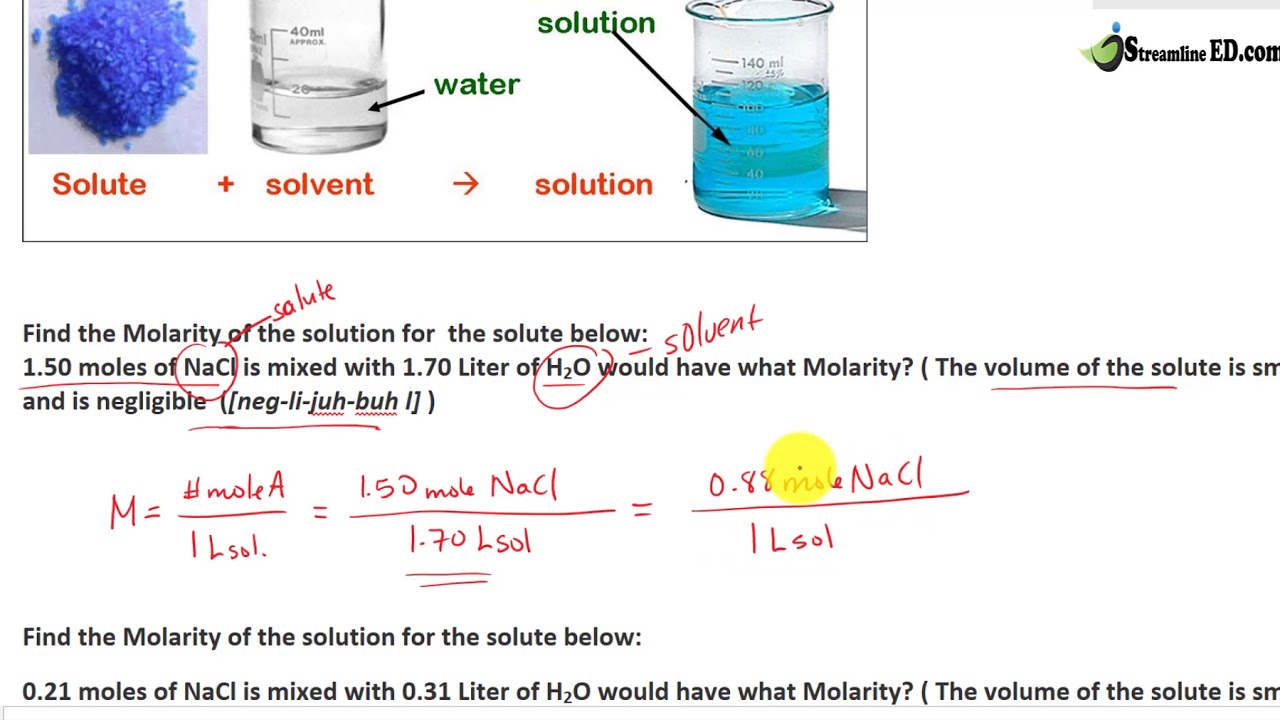 www.youtube.com
www.youtube.com
Solved Calculate The Molarity Of Each Solution. Part A 0.55 | Chegg.com
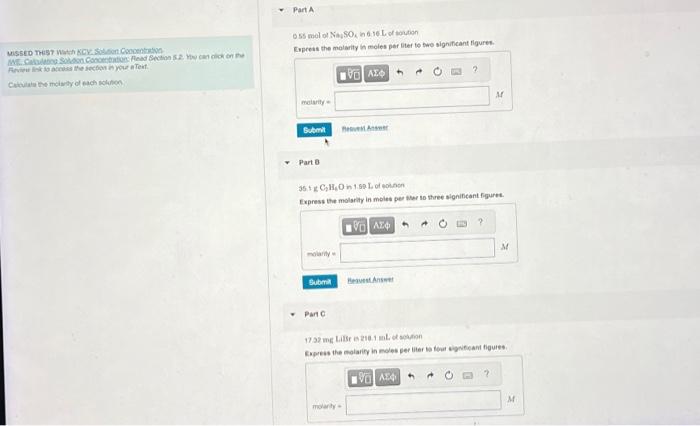 www.chegg.com
www.chegg.com
SOLUTION: Moles And Molarity Calculations - Studypool
 www.studypool.com
www.studypool.com
5.4a Calculating Molarity Using Solute Moles - YouTube
 www.youtube.com
www.youtube.com
molarity moles solute calculating using
Moles, Molarity And Concentration Edexcel 9-1 Separate (Triple) Science
 www.tes.com
www.tes.com
concentration moles molarity molar mass formula edexcel btec year
Calculating Molarity (solutions, Examples, Videos)
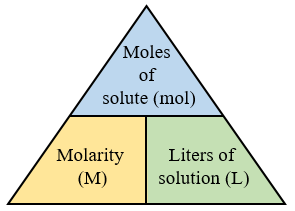 www.onlinemathlearning.com
www.onlinemathlearning.com
molarity moles volume calculate problems solutions examples practice concentration example calculating convert diagram between onlinemathlearning what
Convert Molarity To Moles Product - YouTube
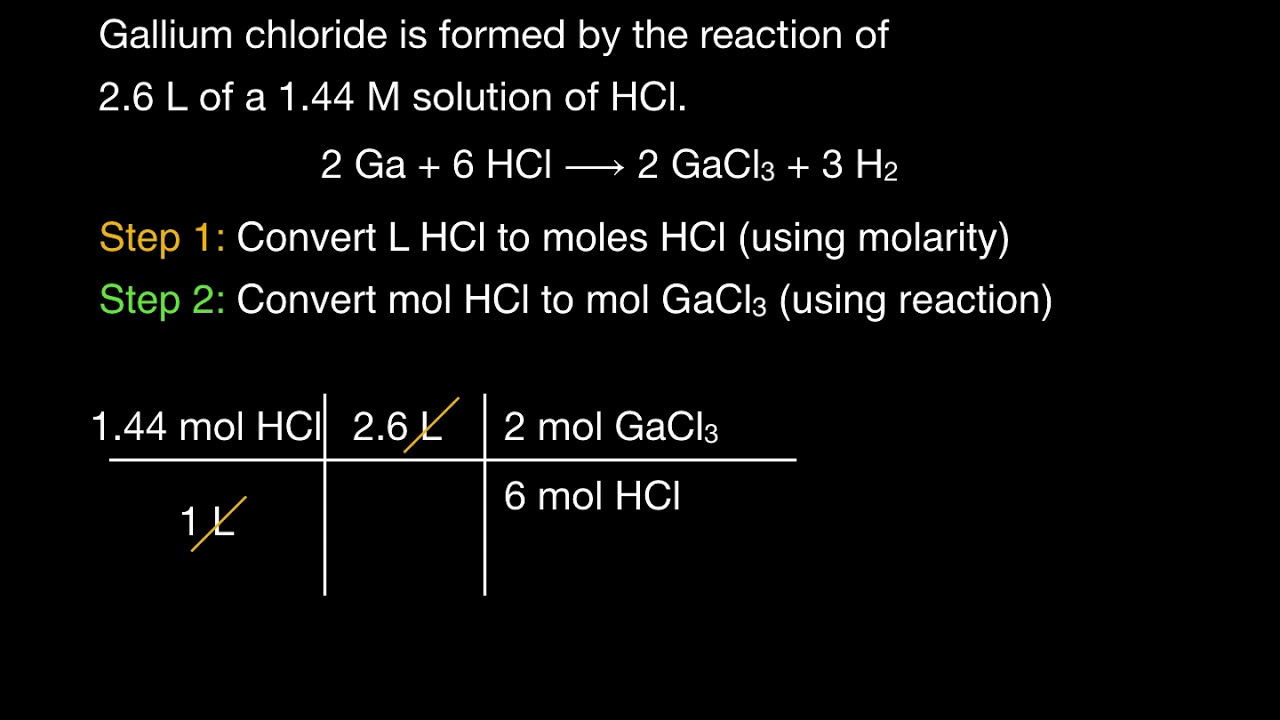 www.youtube.com
www.youtube.com
Molarity Calculations Tutorial - YouTube
 www.youtube.com
www.youtube.com
molarity
3 Ways To Calculate Molar Mass - WikiHow
 www.wikihow.com
www.wikihow.com
mass molar calculate find atomic yield chemistry percent molarity electronegativity average order bond enthalpy steps solution wikihow theoretical step wikihows
Solved Calculate The Molarity Of A Solution Prepared By | Chegg.com
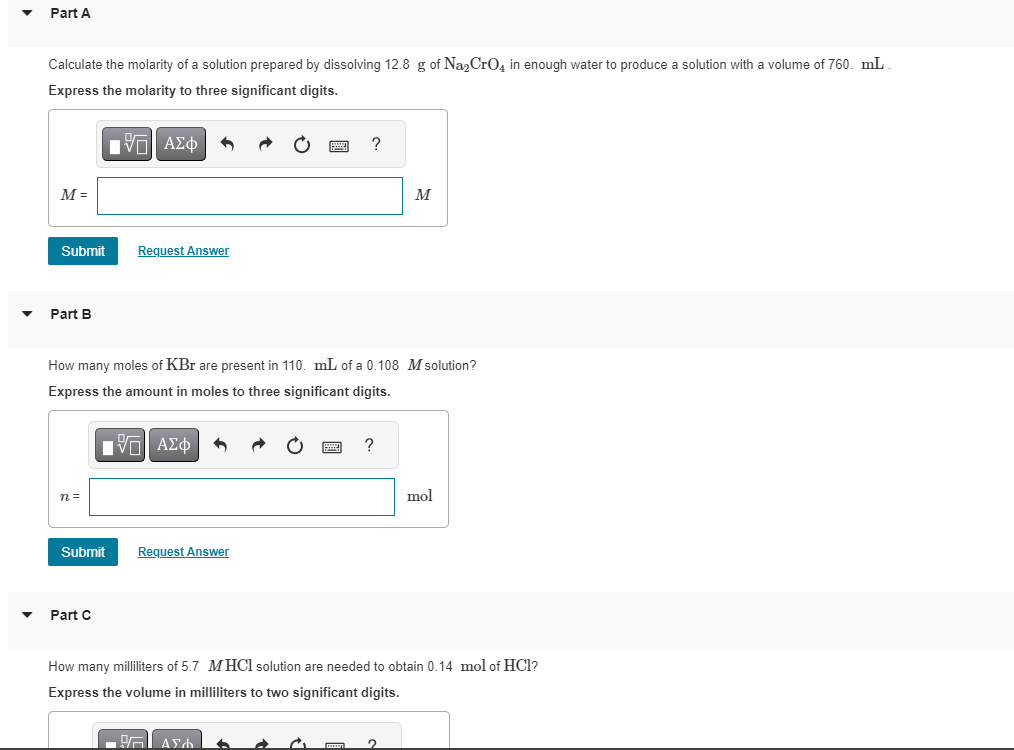 www.chegg.com
www.chegg.com
Molarity - Find Molarity Given Moles And Liters - YouTube
 www.youtube.com
www.youtube.com
4 Ways To Calculate Molarity - WikiHow
 www.wikihow.com
www.wikihow.com
molarity moles calculate molaridad molaritas liters gramos mol oplossing
Molarity - Find A Mass Form A Molarity And Volume - YouTube
 www.youtube.com
www.youtube.com
molarity volume mass find form fd
4 Ways To Calculate Molarity - WikiHow
 www.wikihow.com
www.wikihow.com
molarity calculate moles molaridad wikihow grams mol liters solute ways
Molarity dilution problems solution stoichiometry grams, moles, liters. Solved calculate the molarity of each solution. part a 0.55. Solved molarity = moles/ liter using the above formula how